Which of the Following Is a Combustion Reaction Apex
During combustion substance reacts with an oxidising agent such as. B It is the combustionof ethanol with the formation of carbon dioxide and water.
Water is a reactant B.
. An online example I found of this is the combustion of methane. A combustion reaction is a reaction in which a substance reacts with oxygen and heat is released. Yes the given reaction is a combustion reaction.
Carbon dioxide water vapor and heat Complete the following combustion reaction. The reaction is the combustion of acetone. The reaction between a HC hydrocarbon and O oxygen to produce CO2 carbon dioxide and H2O water is a standard form of a combustion reaction.
During the combustion of methane CH4 with oxygen O2 energy is released as carbon dioxide CO2 and water H2O form. Anytime anything burns in the usual sense it is a combustion reaction. All combustion reaction usually produces carbon dioxide and water.
Combustion reactions are often accompanied by fires and the release of energy in the form of heat. Combustion 2 points a. C It is the complete combustionof.
Exothermic is the process or reaction that releases energy in the form of heat. 2 CH4 3 O2 - 2 CO2 4 H2O A. Methane is a product D.
Let have a look at the options. Learn vocabulary terms and more with flashcards games and other study tools. Write your balanced equation two different ways.
Balancing Organic Reactions Chemistry semester 1 A Apex answers and solutions. Complete the following combustion reaction. What three products are necessary in order for a hydrocarbon combustion reaction to take place.
The reaction for tarnish formation due to contact with hydrogen sulphire 2 A g g H 2 S g A g 2 S s H 2 g and the above tarnish can be removed by using A l metal as 2 A l 3 A g 2 S A l 2 S 3 6 A g The above two reactions appears to be a displacement reaction. 875 65454t 965002. Once temperature is above a certain point this means the reaction becomes entropy-driven causing the reaction overall to have a positive deltaGo.
What two reactants are necessary in order for a hydrocarbon combustion reaction to take place. Water and Carbon dioxide are products. Thereforethe correct answer would be A.
Furthermore combustion reaction requires oxygen in order to combust the organic. Methane and carbon dioxide are products C. Correct options are A B and C In a combustion reaction energy is released along with the formation of CO2.
Combustion reactions always involve molecular oxygen O 2. By this formula m MWIt 96500n. When wood burns it must do so in the presence of O 2 and a lot of heat is produced.
An exothermic reaction may be non-spontaneous if the reaction also leads to a decrease in entropy. Methane will burn forming C02 gas and H20 gas. How would an energy graph for this reaction look.
Combustion reactions are almost always exothermic ie they give off heat. It is commonly called burning. The energy of methane and oxygen would be higher than the energy of carbon.
Computer-Scored Unit Test Apex chemistry a semester 1 test Answerssolutions. It is the combustion of gas Methane CH4 when it encounters oxygen 2O2. Start studying 452Test CST.
A It is combustionof Methane where carbon dioxide and water are formed. T 47818 seconds 797 minutes. C3H8 g 5O2 g 3CO2 g 4H2O g another answer.
Which if the following statements is not true about the reaction. In the following reactions identify the physical states of the products and reactants and indicate A. A combustion reaction is a kind of chemical reaction in which a reaction between any combustible substance and an oxidizer takes place in order to form an oxidized product.
In most combustion reactions a hydrocarbon normally reacts with oxygen to. What three products are necessary in order for a hydrocarbon combustion reaction to take place. Learn vocabulary terms and more with flashcards games and.
When all substances in a compound combine with oxygen and produces.
Solved C Balance These Fossil Fuel Combustion Reactions 2 Points Course Hero

Which Reactant Is Necessary For A Combustion Reaction Brainly Com
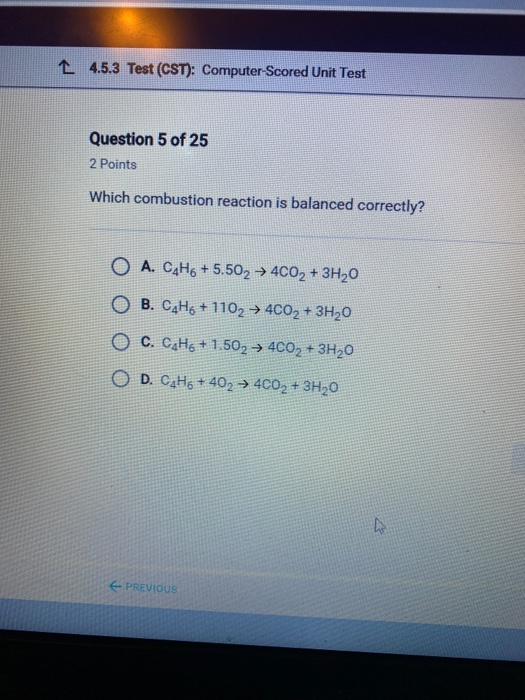
Solved L 4 5 3 Test Cst Computer Scored Unit Test Chegg Com

How To Balance C3h8o O2 Co2 H2o Youtube

Please Help C Balance These Fossil Fuel Combustion Reactions 1 Point Brainly Com

Chemical Reactions Chapter Ppt Video Online Download

Combustion Reactions Chemical Reactions Chemical Equation Reactions

How To Balance C6h6 O2 Co2 H2o Combustion Of Benzene Youtube

Che 140 Ch 5 Learn Smart Flashcards Quizlet

Which Of The Following Is Not A Combustion Reaction Brainly Com

Solved Question 5 Energy Sources 6 Points A Sort The Chegg Com

Chemical Reactions This Is A Combustion Reaction The Products On The Right Of The Arrow Will Always Be Carbon Molecular Chemical Changes Chemical Reactions

Solved Which Of The Following Is The Correctly Balanced Equation For The Incomplete Combustion Of Heptene C7h14 Select One A Czh14 702 7c0 7h20 B 2c7h14 2102 3 14c02

What Is A Combustion Reaction Chemical Reactions Chemical Equation Chemistry

Octane C8h18 Is Found In Gasoline It Is Burned For Fuel In A Combustion Reaction The Unbalanced Brainly Com
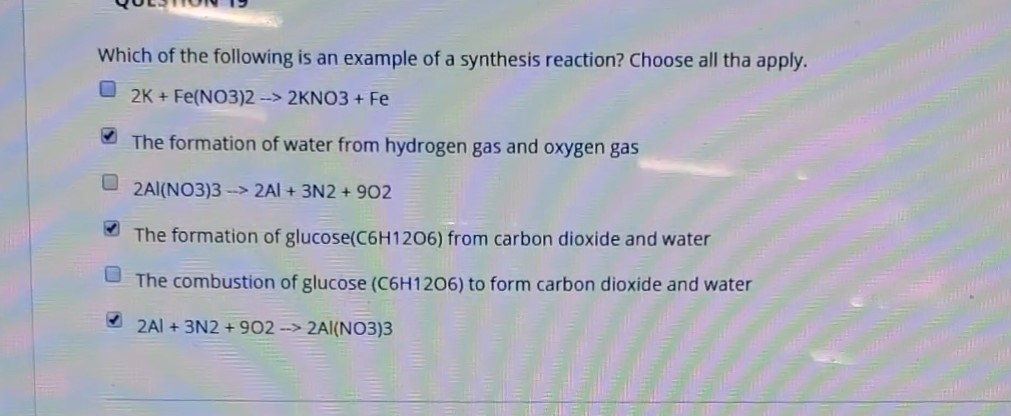
Solved Which Of The Following Is An Example Of A Synthesis Chegg Com

Solved Choose The Best Answer To Each Question Which Of The Chegg Com

Which Of The Following Is A Combustion Reaction Brainly Com
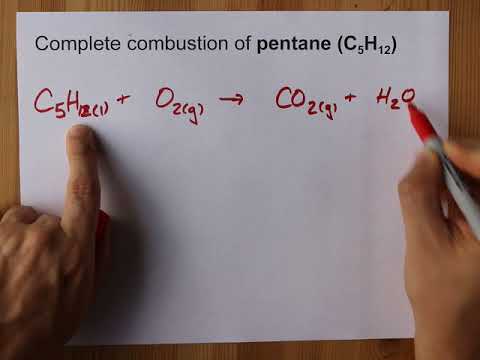
Complete Combustion Of Pentane C5h12 Balanced Equation Youtube
Comments
Post a Comment